March 21, 2010Creating a New State of Matter
Even more surprising, at steady state the material is not in stasis. We are used to seeing matter in familiar static phases: solids, liquids, gases; supercritical fluids, degenerate gases, plasmas, even Bose-Einstein condensates. When viewed at large scales, all these materials sit still when they are in equilibrium. But this phase of matter is something new. It does not sit still. Sand as Maxwell's Demon Ever since a 1999 Physical Review monograph by Jens Eggers, it has been recognized that granular gases can behave as Maxwell's demon: if you shake a partially divided container full of sand with an energy that is not too high, instead of dispersing to both sides, all the sand will tend to move to one half of the container. This phenomenon has foiled high school physics teachers for years. Entropy, Randomness and the Arrow of Time
I have tried to teach my children's science-fair buddies the concept of entropy by having them play this simple Maxwell's demon game: after a short while, it is very obvious to the kids that the power of randomness will mix the two colors of balls soon after they let go of the mouse. Without doing the math, they build a good intuition that in some real sense, it takes a lot of work to separate a mixture and create order. Many physicists believe that the statistical inevitability of rising entropy underlies the arrow of time. Time moves in one direction because our experience moves from a state of low likelihood order to a state of high likelihood disorder. Entropy increases simply because it is tremendously unlikely for it to do anything else. A Failed Experiment And yet if we try to build a physical model of entropy in a gas by bouncing physical beads in a divided box, we will utterly fail to demonstrate the second law. In the real world, all the beads tend to cluster in one side. Curiously, the beads don't always go to the same side every time. Sometimes they will go to one side. Other times they will go to the other side. But unless you shake the beads very very hard, they will not disperse. They will congregate. This is why pendulums and waves are sometimes demonstrated in physics class using huge macroscopic models, but the second law of thermodynamics is not. The experiment with bouncing beads does not work as it is supposed to. It does not teach the "right" lesson.
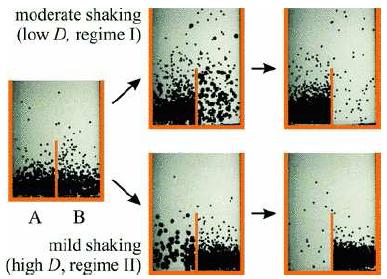
Order appears out of disorder. The arrow of time moves backwards. Phases of a Bi-Disperse Granular Gas Witnessing the reversal of the arrow of time is remarkable by itself. But there are even more intriguing effects. One particularly interesting type of granular matter (studied by Rene Mikkelsen while he was a student of Detlef Lohse) is a granular gas with two different grain sizes. At certain energies the grain sizes will separate from each other. In 2006, Sparisoma Viridi published a stunning discovery with Malte Schmick, and Mario Markus: Mikkelsen's two-grain gas can be energized to a level where the whole mixture stably oscillates from one side of a container to another on a large scale, like a clock. A Viridi clock shows that nontrivial macroscopic behavior can emerge out of a simple energized gas held at constant temperature.
Thanks to studies by Ko van der Weele and others, physicists know that the behavior of a granular gas changes in discrete steps at different levels of energy: at high enough levels of energy, the mixture will behave like an "ordinary" ideal gas; at low enough levels of energy, the mixture will behave like Egger's "Maxwell's demon" and cluster on one side of a container. At some intermediate levels of energy, the particles will tend to separate each other. What Viridi described is a new phase of the gas, somewhere in the middle at appropriate energies and densities, where instead of settling on a stasis, it oscillates forever like a clock. It is stunning to see macroscopic oscillation in a gas that sits at constant temperature. All it takes is negative entropy. Oscillation is the simplest form of computation, and perhaps Viridi's discovery is a hint that a time machine is actually the least interesting thing you can build out of a system with negative entropy. What other complexity can emerge from these systems? The science is very new. Nobody knows where it will lead. Creating New States of Matter in the Dining Room Creating a granular gas requires a bit less equipment than the physics experiments that usually appear in the newspaper. Yet the physics you can do is just as fascinating and novel as the six-billion-dollar collider experiments being done today in Geneva. We decided to do this experiment as part of our elementary school's "science share" month. Five neighborhood kids came over to our house to assemble their own bead-shaking experiments in little plastic capsules. For the third graders, this is just an arts and crafts project. They are very good at arts and crafts. As you can see, by recycling stuff around the house and ordering a few supplies online, my kids and I were able to coax Viridi's surprising new state of matter out of beads and glue and plastic, along with some way to shake it up. What we did was both cheap and easy, and could be reproduced without too much effort in your own living room. Doing it Yourself Here are a few tips if you want to make a granular gas yourself. Beads. The beads we used were ordinary glass beads used for making bracelets. The granular gas works better with more numerous and smaller beads, so for our small beads I used 2.5mm "seed beads." You want enough beads that you can cover the whole bottom of your shaking container with one or two layers. $5.70 worth of beads ordered from Mill Hill would be far more than enough. If you want to mix larger beads, you don't need many. I used 6mm round glass beads. You can get a pile of these for about $4.00 at Uncommon Artistry. Glass beads are cheap and easy to find, but the kids all notice that after few minutes of shaking they acquire a static charge and start sticking to the walls of the container. The experiment works anyway, but if you want to avoid this you might try metal balls - I have not tried them yet, but I just ordered a few dollars of metal balls from Craig Ball Sales. You can get anything online.
Until we built it, we were not sure whether the speaker would be strong enough or would displace enough to shake the beads. But it turns out: no problem - it works. The only issue was that sometimes the tweeter would get unbearably noisy. Terran helped us spend a few days probing the circuitry of the electronics so we could us disconnect the tweeter without disabling the woofer. For our device, this was harder than it seemed at first. Container. This was the hardest part of the experimental setup to figure out. Because I wanted to make some configurations with radial symmetry, I needed a source of lightweight, clear, cylindrical containers. The best thing I found was these plastic mailing tubes at Fast-Pack.com. The smallest quantities I saw were 50, which makes them expensive, although I was lucky and found a box of 100 on closeout sale for $20. You might be able to find them on sale one at a time in a packaging store. The plastic mailing tubes don't have very cleanly-cut ends (I guess they figure that they will be covered by the caps), but especially if you end up using velcro to secure them to the speaker, that does not turn out to be a problem. Walls. Assembling walls inside a 3 inch plastic cylinder that are strong enough to withstand 20 hertz of shaking is a fiddly problem. The solution we settled on was to use two materials. First, we cut the spines off of clear plastic report covers like these from Staples. These can be hot-glued to the inside of the cylinder where we want the walls to be. And you can cut index cards to the desired size and slide them into the spines to make the walls. Our walls go just about an inch off the bottom of the cylinder. Also, we made the bottom of the cylinder out of index cards too. It is important to really attach bottom of the wall to the bottom of the cylinder with no gaps. Otherwise, beads will go through gaps in the bottom of the wall and the effects will be hard to reproduce.
Not only does using Velcro let us quickly swap one experiment with another on the shaker, but it also lets us adjust the cylinders so that they are nice and vertical, even if the bottoms aren't perfectly flat and well-formed. Keeping the cylinder vertical is important for making sure it doesn't resonate sideways and fall apart.
There you have it. Velcro, beads, office supplies, and mailing containers. Add an inquisitive elementary-school mind and a healthy dose of experimental setup. Make your own time machine. I believe there are more opportunities in your kitchen to elucidate recent theoretical physics than anything you can do with rings of superconducting magnets and antimatter. For an excellent survey of the state of granular gas physics and how it relates to Maxwell's demon, read this 2008 paper by Ko van der Weele. Comments
Post a comment
|
| Copyright 2010 © David Bau. All Rights Reserved. |

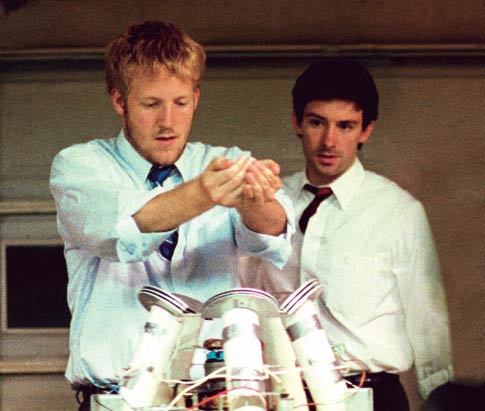
 Velcro. At first, I taped the cylinders down to the speaker cone, which works reasonably well. But three pieces of Velcro (which is easy to obtain at a hardware store) work better.
Velcro. At first, I taped the cylinders down to the speaker cone, which works reasonably well. But three pieces of Velcro (which is easy to obtain at a hardware store) work better.